
Our corporate philosophy is to "contribute to a society where people around the world can maintain their health and maintain hope through the development of innovative new drugs that meet unmet medical needs by pursuing new possibilities for SLC transporters." Based on this philosophy, we will work to create innovative pharmaceuticals to achieve sustainable corporate growth and increase social value.
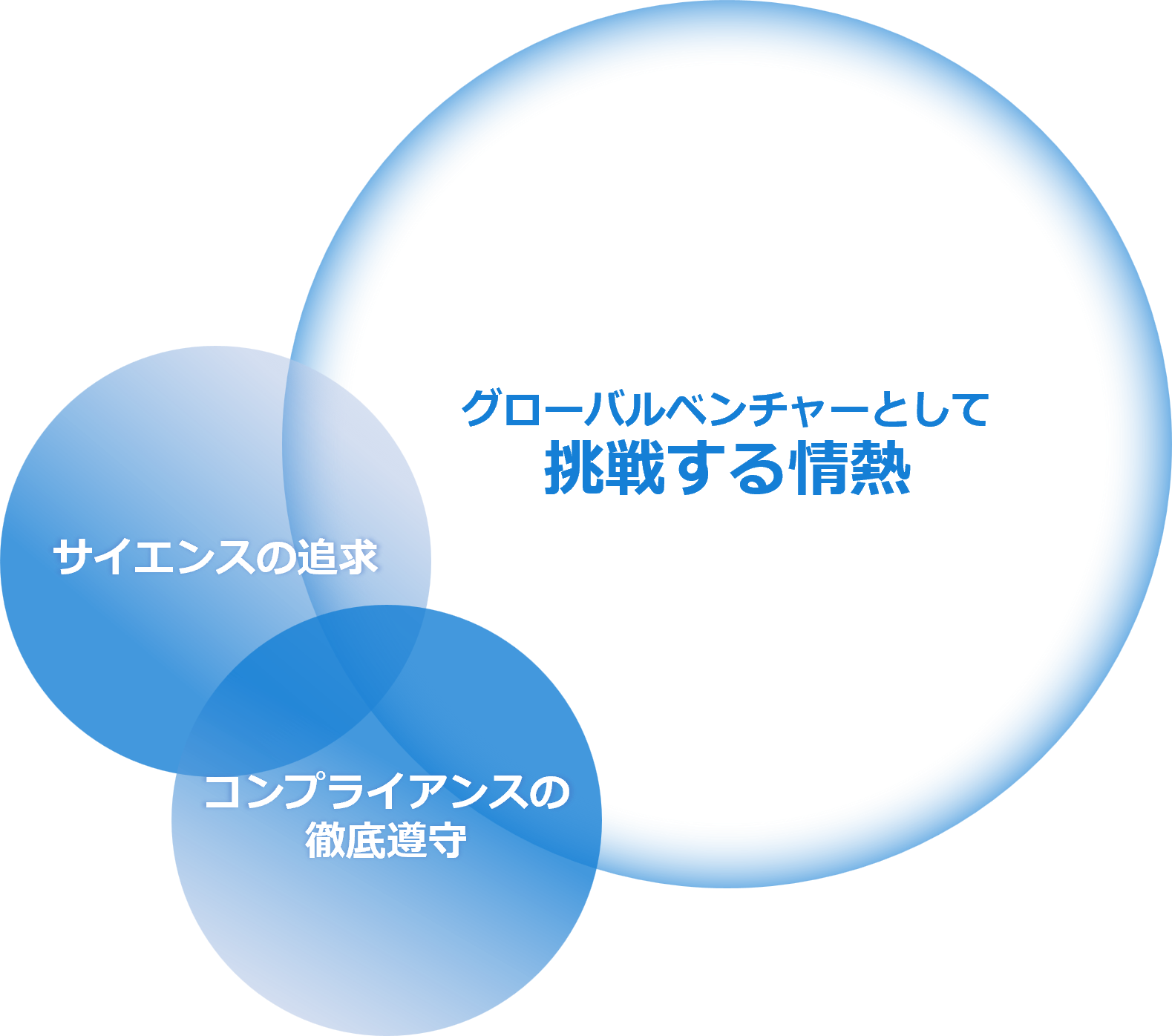
In addition, we have established the following three codes of conduct to realize our corporate philosophy
- Passion to take on challenges as a global venture
- The pursuit of science
- Thorough compliance
We have established the following "Goals for 2030" as a long-term management strategy and are proceeding with step-by-step and steady initiatives.
- LATl inhibitor [(Nambranlat) First-in-Class] received global approval for the treatment of biliary tract cancer as monotherapy Nambran RAT became a blockbuster product from Japan (annual sales of $18 USD)
- Pursuing the potential of new therapeutic drugs focusing on immune mechanisms, expanding into cancer, autoimmunity, and rare diseases
- Clinical Entry of Next-Generation LATl Inhibitors (Best-in-Class)
- Pipeline expansion through the creation of new transporter inhibitors
- Incorporating various drug discovery technologies into a platform to create a system for continuous drug discovery and commercialization

| Name | J-Pharma Co., Ltd |
| location |
〒105-0013 1-10-11 Hamamatsucho, Minato-ku, Tokyo VORT Hamamatsucho II 8th floor TEL: General inquiries (main number) 03-6432-4270 Inquiries from investors (IR Department, Planning Department) 03-xxxx-xxxx FAX: 03-6432-4271 |
| Date of establishment | December 26, 2005 |
| representative | Masuhiro Yoshitake (President & CEO) |
| Capital | 80 million yen (as of March 2025) |
| Fiscal Year | March 31 |
| Business Description | Drug Development |
| Number of Employees | 2025/12 Current O people |
- 40 years of industry experience
- Over 30 years of leadership positions in the global pharmaceutical business at Mega Pharma, three drug products have been approved by the FDA and EMA
- Successful domestic phase 2 of our lead compounds. Obtained FDA agreement and started global phase 3
- 20 years of industry experience
- In charge of global marketing at the consulting firm Mega Pharma
- Increase the value of the startup you founded by 200 times and sell it to a fund
- Led the company in raising a total of7 billion yen in financing.

- He served as the head of the Corporate Strategy Office and the president of the European subsidiary at Shimadzu Corporation.
- Since 2013, he has been appointed as a director of the company, and as an executive officer, he is in charge of sales, financial management, risk management, chief financial officer (CFO) and other areas.
- Since April 2023, he has served as an executive advisor to the company.
- Appointed as an Outside Director of the Company in June 2025



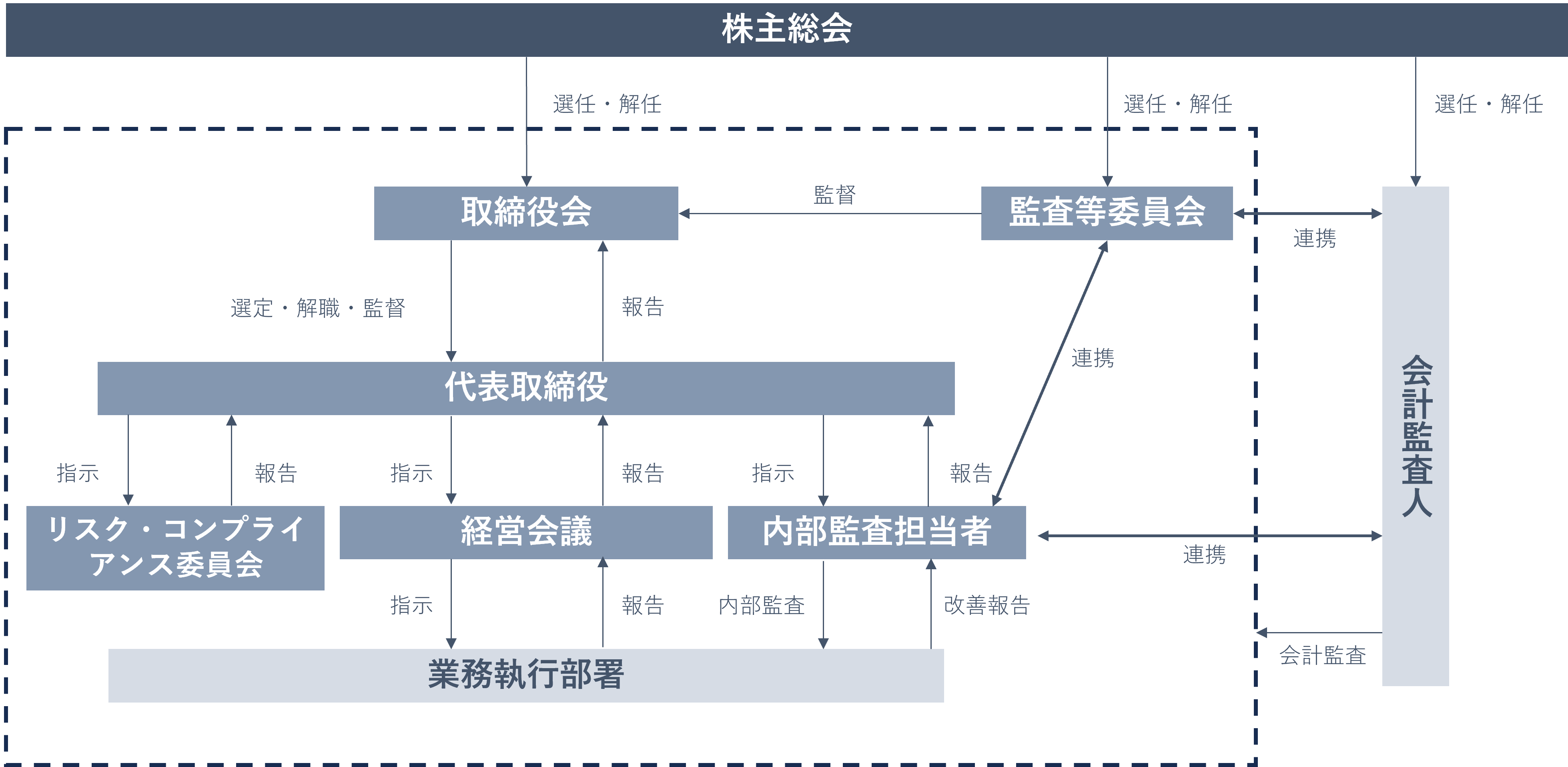
The Company's basic management policy is to ensure management transparency to shareholders and other stakeholders and to continuously increase corporate value by conducting rational and efficient management activities.
The outline of the Company's corporate governance system is as follows.
| December 2005 | Established J-Pharma Co., Ltd. as a company specializing in drug development specializing in cell membrane transporters in Toranomon 1-chome, Minato-ku, Tokyo |
| October 2006 | Adopted the research support project for the practical application of pharmaceuticals and medical devices "Development of drugs for the treatment of hyperphosphatemia" by the National Institute of Medical Sciences |
| October 2006 | Moved the head office to Shinjuku 2-chome, Shinjuku-ku, Tokyo |
| September 2007 | Selected for NEDO Innovation Commercialization Grant Project "Development of Gastric Cancer Therapy" |
| October 2007 | Selected the Ministry of Education, Culture, Sports, Science and Technology's Molecular Imaging Research Program "Development of Novel Molecular Probes Targeting Cancer Cell-Specific Membrane Proteins" |
| August 2009 | Adopted the NEDO Innovation Promotion Project "R&D Project for Novel Phosphorus Adsorbent JPH101 and Implementation of Phase 1 Clinical Trials" |
| August 2010 | NEDO Selected for "Research and Development of Novel Anticancer Therapies Targeting Essential Amino Acid Transporters (LATl) Expressed in Cancer Cells" |
| April 2013 | Selected for NEDO Venture Practical Application Grant Project "Clinical Development of Innovative Anticancer Drugs by Inhibition of Amino Acid Transporters" |
| May 2013 | Moved the head office to Tsurumi-ku, Yokohama City, Kanagawa Prefecture |
| July 2013 | Yokohama City Special Zone Leading Project Grant "Development of in Vitro Diagnostics Kit for Triple Negative Malignant Breast Cancer" Adopted |
| May 2014 | Adopted the NEDO Venture Practical Application Grant Project "Securing POC by Conducting Clinical Trials of the Novel Anticancer Drug JPH203" |
| July 2014 | Yokohama City Special Zone Leading Project Grant "Practical Application of in Vitro Diagnostics for Triple Negative Breast Cancer" Adopted |
| January 2015 | Phase 1 clinical trial of LATl inhibitor Nanbulan RATO (development code: JPH203) in Japan |
| July 2017 | Completed Phase 1 clinical trial of Nambranrat in Japan |
| November 2018 | Phase 2 clinical trial of Nanbulan rat in Japan started |
| April 2019 | Signed an agreement with Ohara Pharmaceutical Co., Ltd. regarding licensing and joint development related to Nanbulan Lato |
| April 2022 | Nanbulan Latte is designated as an orphan drug (orphan drug drug) for advanced biliary tract cancer by the U.S. Food and Drug Administration (FDA) |
| December 2022 | Completed Phase 2 clinical trial of Nanbulan Rato in Japan |
| October 2023 | Development of a centrally transitional LATl inhibitor (development code: JPH034) for multiple sclerosis has been accepted and awarded by the National Multiple Sclerosis Society (NMSS) Fast Forward Commercialization Research Grant Program |
| October 2023 | Established J-Pharma USA to recruit human resources for drug development in the U.S. (liquidation closed in July 2025) |
| June 2024 | JPH034 was selected for the "Drug Discovery Venture Ecosystem Enhancement Project" by the Japan Agency for Medical Research and Development (AMED). |
| September 2024 | U.S. Food and Drug Administration (FDA) Approval for lnvestigational New Drug (IND) Application for Clinical Trials of Nambranrat in Cancer Patients |
| May 2025 | Received a positive response from the U.S. FDA for the CMC (chemistry, manufacturing, and quality control) of Nanbulan Lat, confirming that it meets the quality standards required by the U.S. FDA at the commercial manufacturing scale. |
| June 2025 | Moved the head office to Hamamatsucho, Minato-ku, Tokyo |





Center



Management

University of Medicine

